Visualizing
the navigation of an ensemble of unfolded molecules through the bumpy
energy
landscape in search of the native state
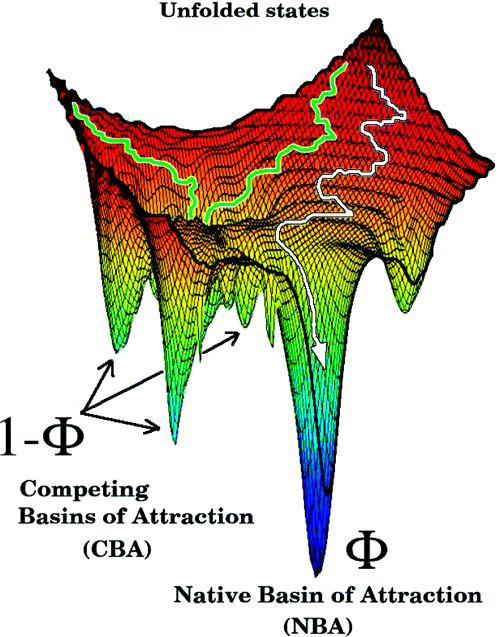
gives a
pictorial view of biomolecular folding. This picture, when
combined with concepts in polymer theory, provides a unified theory of
RNA and protein folding. Just
as for proteins, the major folding free energy barrier for RNA scales
sublinearly with the number of
nucleotides, which allows us to extract the elusive prefactor for RNA
folding. Several folding scenarios
can be anticipated by considering variations in the energy landscape
that depend on sequence, native
topology, and external conditions. RNA and protein folding mechanism
can be described by the kinetic
partitioning mechanism (KPM) according to which a fraction (

) of molecules reaches the native state
directly, whereas the remaining fraction gets kinetically trapped in
metastable conformations. For two-state folders


1. Molecular chaperones are recruited
to assist protein folding whenever

is small.
We show that the iterative annealing mechanism, introduced to describe
chaperonin-mediated folding,
can be generalized to understand protein-assisted RNA folding. The
major differences between the folding
of proteins and RNA arise in the early stages of folding. For RNA,
folding can only begin after the
polyelectrolyte problem is solved, whereas protein collapse requires
burial of hydrophobic residues. Cross-fertilization of ideas between
the two fields should lead to an understanding of how RNA and proteins
solve their folding problems.
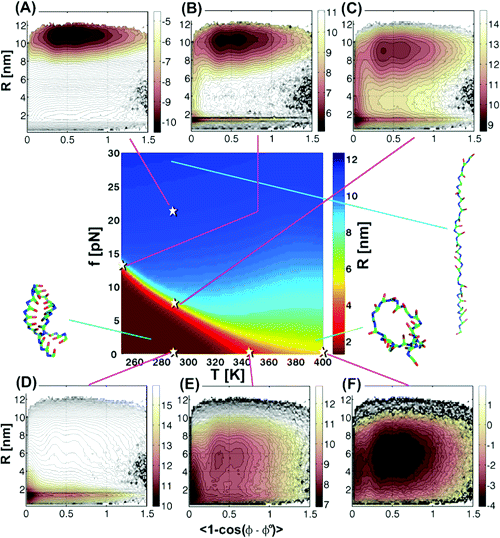
Multiple Probes are Required to Explore and
Control the Rugged Energy Landscape of RNA Hairpins (JACS '08)
Brownian
dynamics simulations, as a function of temperature (
T) and force
(
f)
show that RNA hairpins form by multiple pathways thus revealing the
rugged
nature of the free-energy landscape. While low dimensional free-energy
profiles
can account for some aspects of thermodynamics of hairpin formation
they cannot
account for the observed pathway diversity during the refolding
process. Thus,
a single free-energy surface cannot be used to infer the experimentally
observed
multistate kinetics in hairpin
formation in
nucleic acids. The profound differences between the kinetics of folding
upon
f-
and
T-quench is due to the slow rate of loop nucleation when
the search
for the native conformation commences from stretched conformations as
is the
case upon
f-quench.